摘要

以中空絲膜取出廢水中的氨氮
The extraction of ammonia using membrane contactors provides several advantages in solving a common problem in the wastewater streams of many industries. Some possibilities and limitations of the ammonia removal process will be shown based on experience gained through pilot studies. These pilot systems provide data that will allow scale up to full-sized commercial plants under comparable conditions. With this separation technique, ammonia as a gas species is stripped from an aqueous liquid “feed” phase and captured into an aqueous liquid “receiving” phase in the same membrane device. The membrane used in this process is a microporous hydrophobic hollow fiber, with no inherent selectivity between permeating species. Because the membrane is hydrophobic it can be used to separate the feed phase and the receiving phase; the membrane pores are essentially gas-filled. This paper presents data from pilot trials and a full size plant where commercial membrane contactors are used to remove and recover ammonia from a wastewater stream at an industrial site. Actual waste water from the site containing high concentrations of ammonia was introduced on the shell side and a dilute sulphuric acid solution was introduced on the lumen side of the hollow fibers inside of the modules. Prior to entering the membrane system the wastewater was dosed with a sodium hydroxide solution to raise the pH. Raising the pH increases the partial pressure of free ammonia in the wastewater making it possible to remove. The gaseous NH3 diffuses from the waste water phase across the micro porous hollow fiber membrane wall and reacts with sulphuric acid in the strip solution to form ammonium sulphate.
INTRODUCTION
Dissolved gases like NH3, H2S or NOx in waste water lead to contamination in the sewage system and high treatment costs for municipal waste water treatment plants. This translates into high penalty fees that are paid by the company discharging these contaminates into the sewage stream. In many cases a membrane-based water treatment system can be justified because of a favorable pay back time. Large-scale processing using conventional treatment processes such as extraction, stripping or absorption can lead to several problems or issues. For example, a stripping column has a relatively large footprint and is energy intensive due to complete pressure loss of the treated water. In addition, a secondary striping unit is needed to clean up the striping media air before the air is vented to the atmosphere. Such issues may be overcome by using an alternate solution called Membrane Contactor technology. Membrane Contactors can remove ammonia from waste water and recover it to a usable form in a single step. It is therefore an adequate and desirable solution for treating the ammonia waste water without polluting the air.
Removal of ammonia using hydrophobic hollow fiber membranes in Membrane Contactors has been tested on small pilot systems in the past. There is currently very limited data available on large scale field installations. Because there is limited data there is insufficient knowledge about how to design and size full scale systems. Operating parameters such as waste water flow rate, pH, temperature, and the ammonia concentration will significantly impact the ammonia removal characteristics and removal efficiency. Generating additional data is therefore an important step towards developing and demonstrating this technology.
PROCESS DESCRIPTION
Ammonium ion (NH4+) in water reacts with hydroxide ion (OH-) according to the following Equation 1.
NH4+ + OH− ↔ NH3 (g) + H2O (l) Eq 1
This reaction is reversible and can be driven forward or backward depending on the water pH as shown in Figure 1.
Fig.1. Solubility of Free Ammonai Based on pH and Temperature
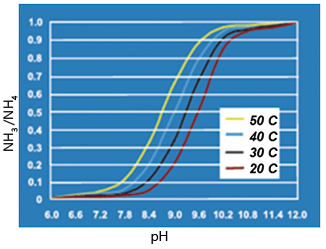
At a pH of 11.3 or higher, the equilibrium favors the formation of free ammonia gas which can be removed from a waste water solution across the air filled pores of a microporous hydrophobic membrane when a proper driving force is maintained. The small pore size and the hydrophobic nature of membrane prevents the liquid phase from entering into the pores or flowing through the porous wall due to surface tension effect. Because of the very low Henry constant and high solubility of NH3 compared to other dissolved gases in water (e.g., CO2 or O2), the free ammonia gas will be difficult to remove by applying vacuum or sweep gas-vacuum combination as in typical degassing operations with Membrane Contactor technology [2]. However, an acid solution will work very effectively as a means of removing the ammonia gas from waste water. The low-pH sulphuric acid solution will instantly react with ammonia gas according to Equation 2 below to form ammonium sulphate. This will generate and maintain the concentration differential or driving force for removing ammonia from waste water.
2NH3 + H2SO4 ↔ (NH4)2SO4 Eq 2
The process above generates a concentrated solution (up to 30%) of ammonia sulphate, which is a fertilizer. This process, also described as: “TransMembraneChemiSorbtion” (TMCS) [3], is shown schematically on a commercial available hollow fiber Membrane Contactor Modul as well as on a single fiber in Figure 2. The wastewater flows through shellside of contactor (outside of the membrane), while the acid solution (ex. sulphuric acid) is circulating on the lumenside.
Fig. 2. TMCS of ammonia using Liqui-Cel® Membrane Contactors

System design and measurement setup
In 2002 Membrana GmbH in Wuppertal Germany installed a TMCS pilot plant to test the removal of ammonia from waste water with the purpose of reducing waste water disposal costs at the manufacturing plant. In the first step, a system utilizing two 6x28 Liqui-Cel® Contactors (2x1 system configuration) with X-50 Celgard® polypropylene hollow fiber membrane was installed to treat 0.5-1 m³/hr of waste water. Later, the system was expanded with a third contactor containing an X-40 Celgard® membrane. Because X-40 has lower porosity than X-50 membrane it was chosen for possible impact on water vapor transport through the membrane.
After it was determined that X-50 still provided superior ammonia stripping performance and successful trials on the 6inch system the plant was redesigned in 2004 to operate up to 30 m³/hr with a 2x1 system utilizing 14x28 Liqui-Cel Contactors with X-50 fiber.
The waste water stream consisted of two inlets with different NH3 concentrations and temperatures so that different test cycles could be run. The different system sizes (6-inch and 14-inch) where fully adjustable so that configuration changes from 1x2 to a 2x1 system were possible. The general system design with two contactors in series is illustrated in the following Figure 3.
Fig.3. Basic P&ID of a TMCS system with two contactors in serial [4]
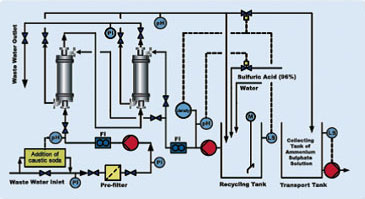
The ammonia measurement during the pilot trials was done with the Kjeldahl method in the laboratory. Later, when the 14-inch system was operational, the ammonia measurement was occasionally completed with an ion sensitive NH4-N-Elektrode (Nadler MiniCal - online measurement). The compact measurement technique includes a flow-through cell with pH adjustment from high-pH caustic water to pH of 5 to fully react incoming NH3 into NH4, which is measured immediately and online. After validation of the system the concentrations were measured only on the inlet to the municipal water.
The measurement setup for ammonium sulphate was done by density control of a first recirculation tank for the process acid. A second tank was installed for storing the high content ammonia sulphate solution. Depending on the filling level, the fluid was pumped from the first tank to the second tank.
The pH as major control variable was recorded for all inlet and outlet samples from each contactor. For the waste water phase, the inlet pH was controlled by adjusting the caustic soda (NaOH) dosage. The acid phase inlet pH was controlled by adjusting the sulphuric acid (H2SO4) dosage.
While the inlet temperature, pressure and flow rate were dependant on waste water and production parameters, the acid circulation could be adjusted by process control. During pilot trials and later in the plant, the acid flow was adjusted automatically to one third of the waste water flow as this flow ratio was found to be the most optimum.
Results
The goal of the Liqui-Cel® Contactor TMCS system was to remove 90% of the ammonia at the Membrana GmbH plant site. The performance of the system has exceeded expectations with a removal rate of 95% [5].
Some of the dependencies are shown for the 6-inch and 14-inch systems with two contactors in series in the following figures to demonstrate how the process can be influenced and where process optimization might be obtained. During evaluation the NH3 inlet concentration was approximately 1300 ppm with minimum to maximum inlet concentration of 600-2500 ppm.
As already shown in figure 1 the pH and temperature of the waste water influences the free ammonia content. Since only free ammonia gas can be removed by the hydrophobic membrane, the pH and temperature have a direct influence on the obtainable removal rate. In Figure 4 the ammonia removal is therefore shown as a function of the waste water pH and the flow rate. The waste water temperature was relatively constant at 45-50°C.
Fig.4. NH3 removal dependence on waste water pH
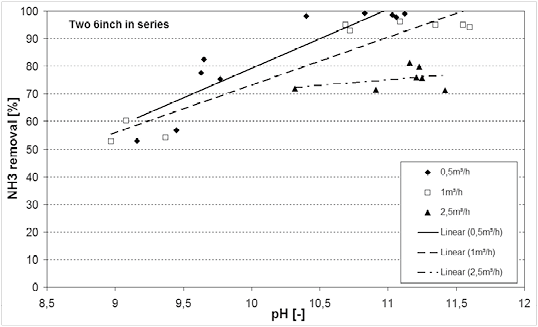
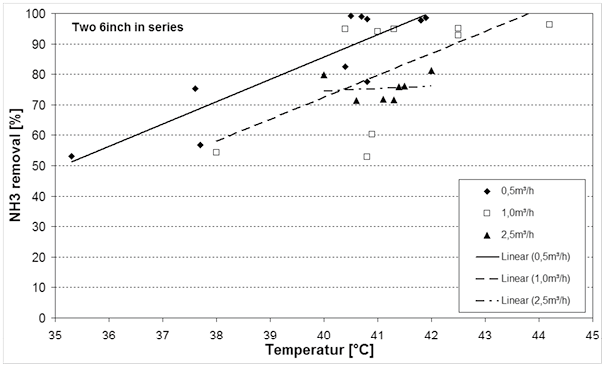
Figure 5 shows the ammonia removal at different temperatures and flow rates while pH was controlled in the range of 10 to 11.5. The maximum process water temperature was limited to 50°C because of the limitation of the membrane contactor housing materials. Also, as mentioned above, as temperatures increase, the amount of water vapor passing through the membrane also increases. This dilutes the acid solution thereby increasing H2SO4 consumption.
Fig.5. NH3 removal dependence on waste water temperature
During piloting it could be shown that for a 6-inch Contactor with a membrane area of 42 m², the performance was best at a flow rate between 0.5 m³/hr and 1 m³/hr. Figure 6 illustrates NH3 removal at different waste water flow rates with two 14-inch contactors in series while one 14-inch Contactor has a membrane area of 220 m². In this period, the temperature of 50°C and the pH of 10-11.5 were held relatively constant.
Fig.6. NH3 removal dependence on waste water flowrate
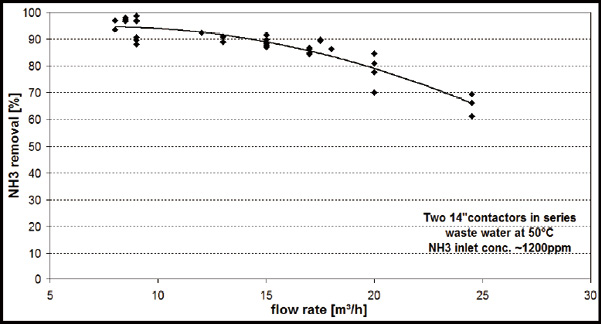
When looking at the ratio of membrane area of 6-inch (42 m²) compared to 14-inch (220 m²) no typical water flow per membrane square meter could be found. This made scaling up and down for system sizing and determining the most economical option difficult. On future systems, connecting additional contactors in parallel or in series for process optimization is possible but more testing is required to obtain actual data.
The acid and caustic consumption depends on the inlet water, the pH, the water temperature and the ammonia content. When the inlet parameters are closest to being in the free ammonia state, with concentrations suitable for membrane contactor treatment and that fit within the operating window of the TMCS process, less acid and caustic will be required.
During the trials the generated ammonia sulphate concentration was up to 30%, which was of very good quality, but still with pH<4. So before it could be used as fertiliser it was processed in an evaporation column to increase the concentration. The waste water after the TMCS plant with pH<10 was sent directly to the municipal water system.
Summary
It can be stated that the process works as expected even in large scale applications. As seen in the summary Table 1 below, a 95% NH3 removal was achieved.
|
Process Parameter |
Range of typical values (1x2) 6-inch system |
Range of typical values (1x2) 14-inch system |
|---|---|---|
|
Ammonia concentration waste water |
500 to 2500 mg/L |
500 to 2500 mg/L |
|
Waste water temperature |
45 to 550C |
45 to 550C |
|
Waste water flow rate |
0.5 to 1 m3/hr |
5 to 10 m3/hr |
|
Adjusted waste water pH |
pH>10.5 |
pH>10.5 |
|
Acid strip solution flow rate |
0.2 to 0.4 m3/hr |
1.5 to 5 m3/hr |
|
Strip solution pH |
pH<2 |
pH<2 |
For new systems, we recommend additional pilot tests in order to generate more data with varying inlet conditions. Limited system sizing based on the findings of these trials is possible but more research is necessary to define process limitations and performance parameters in a wider range of operating conditions.
(All written information, photos, and illustrations are copy write© 2011 Membrana-Charlotte, a Division of Celgard LLC. Liqui-Cel® is a registered trademark of Membrana-Charlotte, a Division of Celgard LLC.)
參考文獻
- Daniel Waterkamp: Praktikumsbericht – Thema: Entfernung von Ammoniak mittels Membrantechnologie; internal report for Membrana GmbH, 2002.
- Fred Wiesler: Membrane contactors an introduction to the technology; Ultrapure Water, UP130427, 1996.
- Jorge Munoz: Process description of NH3 removal; Membrana-Charlotte, internal manuscript, 2002.
- Maria Stasiak: Interne Versuchsberichte zur TransMembranChemieSorption, Membrana GmbH, Wuppertal, 2000.
- Membrana-Charlotte: Tech Brief Nr. 43 – Successful Ammonia Removal from Wastewater Using Liqui-Cel Membrana Contactors at a European Manufacturing Facility; http://www.liqui-cel.com, 2007.









留言(0)